Regulatory bodies in Europe and beyond are working together to prevent shortages of medicines and limit their possible impact. The reasons for such a shortage can be various factors, including increased demand during various emergency situations.
The European Medicines Regulatory Network aims to minimize the impact of drug shortages on patients. One of the ways to solve this problem is to provide pharmacies with a temporary opportunity to manufacture medicinal products for which there is a shortage. The European Pharmacopoeia has developed a methodology for determining and creating the first list of pharmaceuticals for which there is or may be a shortage.
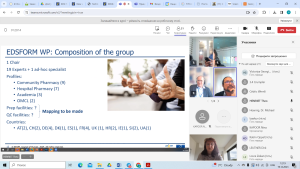
A working group of the European Pharmacopoeia was created to develop monographs for the temporary production of unregistered medicinal products in pharmacies.
The first meeting of the working group was held on December 19, 2023. The head of the Department of Pharmaceutical Chemistry , Professor Viktoriya GEORGIYANTS became a member of this group from SPhU and took part in its work.
Other members of the working group represent Great Britain, Denmark, Ireland, Spain, Germany, Slovenia, France, Croatia and Switzerland.
The participants were presented with the methodology to determine the risk of a shortage in Europe in the near future and predetermined a plan for the development of temporary monographs for these medicinal products.